Anorexia nervosa
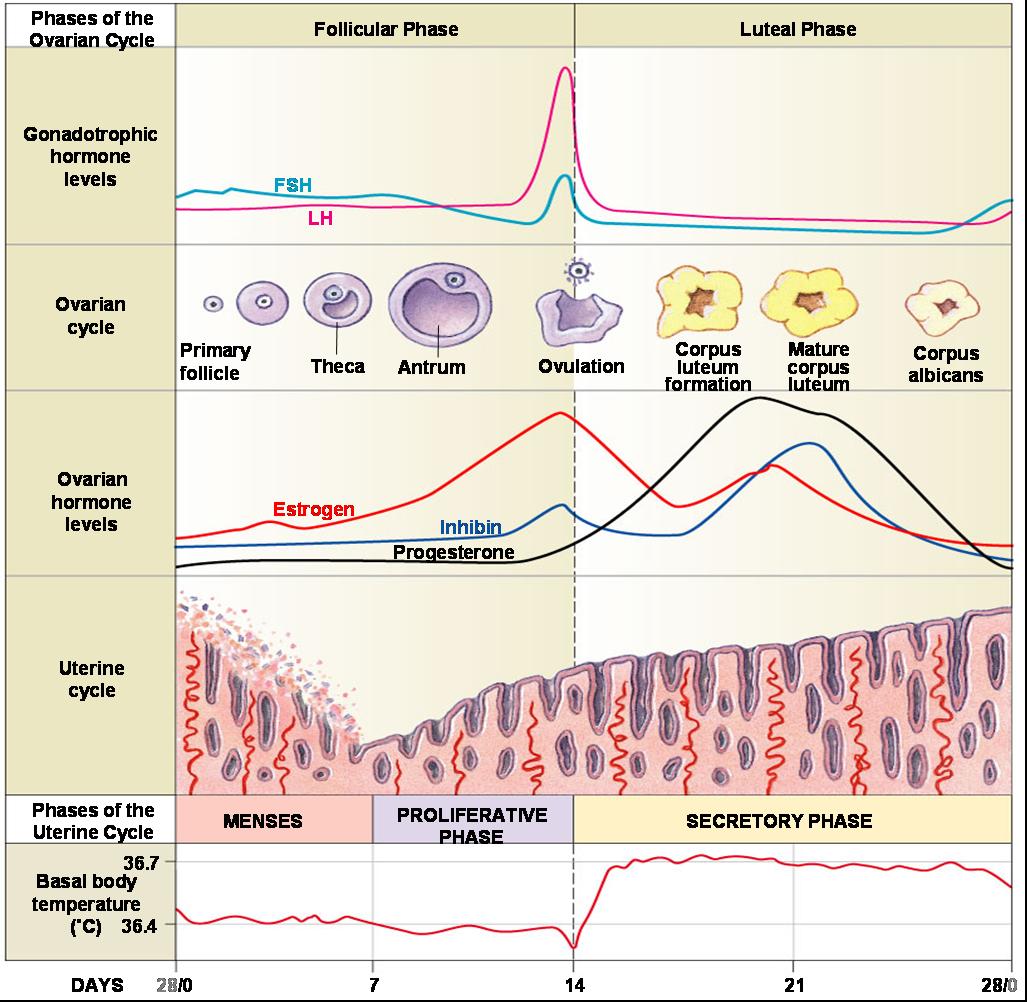
In past editions of the DSM, amenorrhea was listed as a criterion for AN. While it is not included in the DSM-5, the absence of menstruation for >3 months affects about 66-84% of women with AN, while an additional 6-11% of women report light or infrequent menses (oligomenorrhea). Low BMI, high levels of exercise, and low caloric intake are the strongest predictors of amenorrhea. Amenorrhea occurs because food deprivation inhibits the hypothalamic-pituitary-gonadal (HPG) axis, decreasing the release of gonadotropin-releasing hormone (GnRH) from the hypothalamus, which subsequently decreases the release of follicle stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary gland, which in turn, decreases the synthesis of estrogen and progesterone. A functioning HPG axis is imperative for ovulation and, thus, fertility.
In past editions of the DSM, amenorrhea was listed as a criterion for AN. While it is not included in the DSM-5, the absence of menstruation for >3 months affects about 66-84% of women with AN, while an additional 6-11% of women report light or infrequent menses (oligomenorrhea). Low BMI, high levels of exercise, and low caloric intake are the strongest predictors of amenorrhea. Amenorrhea occurs because food deprivation inhibits the hypothalamic-pituitary-gonadal (HPG) axis, decreasing the release of gonadotropin-releasing hormone (GnRH) from the hypothalamus, which subsequently decreases the release of follicle stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary gland, which in turn, decreases the synthesis of estrogen and progesterone. A functioning HPG axis is imperative for ovulation and, thus, fertility.
Recent research has found that estrogen receptor alpha (ERα), a nuclear receptor activated by estrogen, is also involved in the nutritional regulation of reproduction. Caloric restriction has been shown to decrease liver ERα activity, which disrupts the estrous cycle. However, dietary amino acids prevent this from occurring by promoting hepatic ERα activity. Finally, increased physical activity (over-exercise) is sometimes seen in patients with AN. It's been found that moderate exercise leads to increased fertility; however, exercising to exhaustion leads to a 2.3- to 3-fold increased risk of infertility. This effect of exercise is thought to be independent of body fat stores.
What is the upshot of this? According to a survey by Hassan and Killick, it takes underweight women (BMI <19 kg/m2) 4 times longer to conceive than it does for women with a normal BMI. Specifically, they found that underweight women took an average of 29 months to become pregnant, compared to 6.8 months for women of normal weight.
What is the upshot of this? According to a survey by Hassan and Killick, it takes underweight women (BMI <19 kg/m2) 4 times longer to conceive than it does for women with a normal BMI. Specifically, they found that underweight women took an average of 29 months to become pregnant, compared to 6.8 months for women of normal weight.
Bulimia nervosa
About 7-40% of women with BN report amenorrhea and 36-64% report oligomenorrhea. Research has found that women with BN have increased odds of receiving fertility treatments. This is thought to be possibly related to increased rates of polycystic ovarian syndrome (PCOS) in women with BN. The pathophysiology of infertility in PCOS is complex, but strongly associated with insulin resistance, which will be discussed in more detail below.
Binge eating disorder
While amenorrhea and oligomenorrhea may not be surprising consequences for patients with AN or BN, patients with BED have also been found to have light, infrequent, or absent menses, even after controlling for conditions like PCOS, or compensatory behaviors like purging. Obesity has been shown to increase the risk of infertility, miscarriage, poor pregnancy outcomes, and impaired fetal well-being. In fact, it's been found that with each unit increase in BMI above 29 kg/m^2, the chance of spontaneous conception decreases by 5%. Studies have found that obesity impairs the HPG axis, and also affects oocyte quality and uterine receptivity.
This decreased fertility in obese women is thought to be multifactorial. Elevated serum free cholesterol concentrations have been associated with reduced fecundity, and dyslipidemia is common in obese patients. Further, abnormal levels of insulin, leptin, and adiponectin in obese patients have been shown reduce fertility.
In obese patients with BED, insulin levels rise, leading to insulin resistance. This insulin resistance is highly correlated with PCOS. High levels of insulin inhibit synthesis of sex hormone-binding globulin (SHBG), leading to increased free testosterone levels, and also increase ovarian androgen synthesis. Insulin resistance has been shown to lead to abnormally high levels of LH. This combination results in a hyperandrogenic state that suppresses ovulation and thus reduces fertility.
Leptin is a hormone produced by adipose tissue that normally works to maintain energy homeostasis by inhibiting hunger/ reducing food intake and regulating pancreatic islet cells. Levels are low in women with AN, but markedly increased in obese patients, who develop a leptin resistance. Leptin affects reproduction by facilitating GnRH secretion in the HPG axis. Leptin-deficient female mice produce low levels of gonadotropins like LH and FSH, and, consequently, sex steroids, which partly explains the hypogonadotropic hypogonadism in patients with AN. However, high concentrations of leptin, like those seen in obese patients with BED, have been demonstrated to directly interfere with estradiol production and oocyte maturation, which also decreases fertility.
Levels of adiponectin, another hormone secreted by adipose tissue, are low in obese patients. Adiponectin has insulin-sensitizing, anti-atherosclerosis and anti-inflammatory effects, but affects female fertility by stimulating granulosa cell steroidogeneis, and by possibly playing a role in uterine receptivity and embryo development.
This decreased fertility in obese women is thought to be multifactorial. Elevated serum free cholesterol concentrations have been associated with reduced fecundity, and dyslipidemia is common in obese patients. Further, abnormal levels of insulin, leptin, and adiponectin in obese patients have been shown reduce fertility.
In obese patients with BED, insulin levels rise, leading to insulin resistance. This insulin resistance is highly correlated with PCOS. High levels of insulin inhibit synthesis of sex hormone-binding globulin (SHBG), leading to increased free testosterone levels, and also increase ovarian androgen synthesis. Insulin resistance has been shown to lead to abnormally high levels of LH. This combination results in a hyperandrogenic state that suppresses ovulation and thus reduces fertility.
Leptin is a hormone produced by adipose tissue that normally works to maintain energy homeostasis by inhibiting hunger/ reducing food intake and regulating pancreatic islet cells. Levels are low in women with AN, but markedly increased in obese patients, who develop a leptin resistance. Leptin affects reproduction by facilitating GnRH secretion in the HPG axis. Leptin-deficient female mice produce low levels of gonadotropins like LH and FSH, and, consequently, sex steroids, which partly explains the hypogonadotropic hypogonadism in patients with AN. However, high concentrations of leptin, like those seen in obese patients with BED, have been demonstrated to directly interfere with estradiol production and oocyte maturation, which also decreases fertility.
 |
| Under physiologic conditions, leptin reduces hunger and food intake. The mouse on the left was leptin-deficient. |