Last week news shows such as Today
announced results from a recent study from the National Institute of Child Health and
Human Development that associates
caffeine consumption with early pregnancy loss. Headlines stated “drinking three or more caffeinated beverages a day raised
the risk of early pregnancy loss.” These
headlines further confuse the already confusing question about how much
caffeine intake is “okay” if you are trying to conceive or already pregnant.
The study published in Fertility and
Sterility followed 344 expectant couples and examined lifestyle factors and the
rate of early pregnancy loss. The study measured the number of caffeinated
beverages partners drank as well as multivitamin use before they conceived through the
seventh week of pregnancy. Researchers concluded that drinking three or more
caffeinated beverages a day (before conception or during pregnancy) raised the
risk of early pregnancy loss by 74 percent. Male preconception consumption of
caffeinated beverages was found to be just as strongly associated with
pregnancy loss as females. The study however found that if a woman took multivitamin while
she was trying to become pregnant through the first seven weeks of
pregnancy there was nearly a 80 percent reduction in the risk of miscarriage.
The study however only looked at an
association, meaning it doesn't prove a cause and effect relationship. It does not prove that caffeine intake itself
leads to miscarriage. Another limitation is that the study examined the number
of caffeinated beverages rather than measure total caffeine intake. Caffeine content of caffeinated beverages can vary wildly between beverages. Nor did the study control for other confounding factors
(exercise, sleep, or recreation drug use). Individuals who drink more then 3 cups of
coffee a day may be different that those who do not drink coffee. If these
factors are not controlled for results can be misleading. For instance, high
caffeine drinkers may have higher levels of subjective stress with poorer sleep habits etc.
which may contribute to fertility loss. Cigarette
smoking, alcohol consumption, and a less health conscious lifestyle have
all been linked to increased coffee consumption .
A previous 2011 systematic review published in Birth Defects
Research Part B of Developmental and Reproductive Toxicology examined both human and animal studies and he risk of spontaneous abortion from caffeine
exposure. They concluded there was fair
to good evidence that consumption of caffeinated beverages during pregnancy at
a level ≤5 to 6 mg/kg body weight/day does not increase the risk of spontaneous
abortion. Very very high caffeine intake in some animal studies demonstrates
some increased risk however woman drinking
over a dozen cups of coffee in a
day would not approach this level of caffeine intake.
Previous publications report an association between
caffeine use in pregnancy and low birth weight and preterm birth. For instance a 2014 meta-analysis of 100,000+ women reported that increases in maternal
caffeine intake during pregnancy is associated with increased risk of
delivering low birth weight infants (in a dose dependent manner). However a randomized double blind trial that
analyzed the effect of reducing caffeine intake found no effect on
birth weight or preterm birth when
caffeine intake was decreased by 50% in women drinking 3+ cups a day prior to study
enrollment. A 2010 systematic review similarly
did not demonstrate a significant association between maternal caffeine intake anytime
in pregnancy and preterm birth.
Given the knowledge of literature the American College of Gynecology states there is insufficient evidence to support reducing caffeine use below
200mg/day.
For hopeful or expecting mothers the first step is to determine
how much caffeine you are consuming.
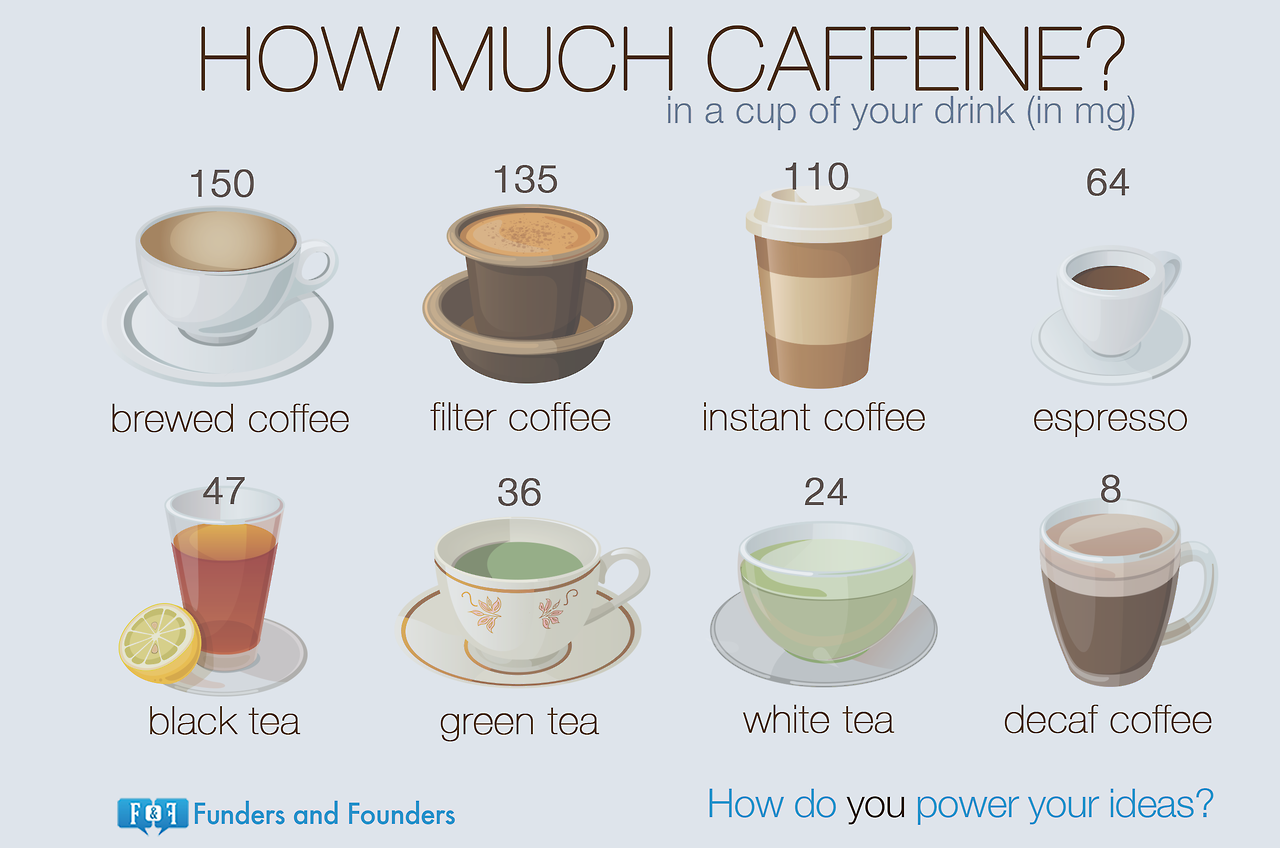
Caffeine is most associated with coffee, tea, soft drinks, and energy
drinks. Content and can vary dramatically between products and brands. For example the same size coffee at Starbucks
has double the caffeine content compared to McDonalds.
Caffeine can be also be present in unexpected places
such some prescription and over the counter medications such as those for
flu/cold, allergy, and headaches as well as diet pills and diuretics. Caffeine content of popular items can be
found here.
Dr. Williams of the Albert Einstein College of
Medicine and a spokesman for the American College of Obstetricians and
Gynecologists warns " what I do end
up seeing not infrequently, an effort to really be as thorough as possible, a
lot of women will go cold turkey on caffeine. And what ends up happening is
invariably these women will then develop rebound headaches and take medications
to treat the headaches. Those medications may be harmful.”
If you decide to cut caffeine “cold turkey” you may experience
withdrawal symptoms such as headache,
anxiety/irritability, constipation/diarrhea, low mood, low energy, sweating, or
shakiness. Most symptoms dissipate in few days but can last as long as two
weeks for heavy drinkers. Gradually decreasing caffeine content over 1-2 weeks
can minimize the risk of withdrawal symptoms. Strategies for decreasing caffeine content include transitioning to decaf, switching out tea for coffee, or drinking tea with a lower caffeine content.